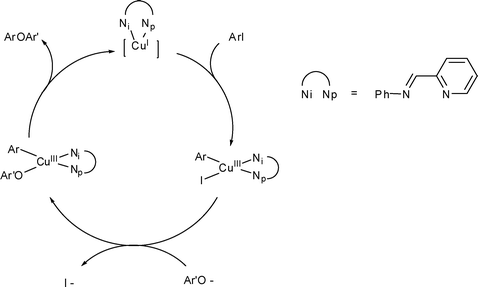
In the Ullmann condensation or Ullmann-type reaction is the copper-promoted conversion of aryl halides to aryl ethers, aryl thioethers, aryl nitriles, and aryl amines. These reactions are examples of cross-coupling reactions.
Ullmann-type reactions are comparable to Buchwald-Hartwig reactions but usually require higher temperatures. Traditionally these reaction requires high-boiling polar solvents such as N-methylpyrrolidone, nitrobenzene, or dimethylformamide and high temperatures (often in excess of 210° C) with stoichiometric amounts of copper. Aryl halide were required to be activated by electron-withdrawing groups. Traditional Ullmann style reactions used "activated" copper powder, e.g. prepared in situ by the reduction of copper sulfate by zinc metal in hot water. The methodology improved with the introduction of soluble copper catalysts supported by diamines and acetylacetonate ligands.
Goldberg reaction: C-N coupling
Aryl iodides are favored arylating agents. The catalyst used is formed from copper(I) iodide and phenanthroline. As this reaction proceeds well with an electron-rich aryl iodide it is a valuable alternative to the Buchwald-Hartwig amination reaction, which gives best yields with electron-poor aryl halides. The scope is extended to amides.
|
