Organic Synthesis
EliminationRxns
| Mechanism:
|
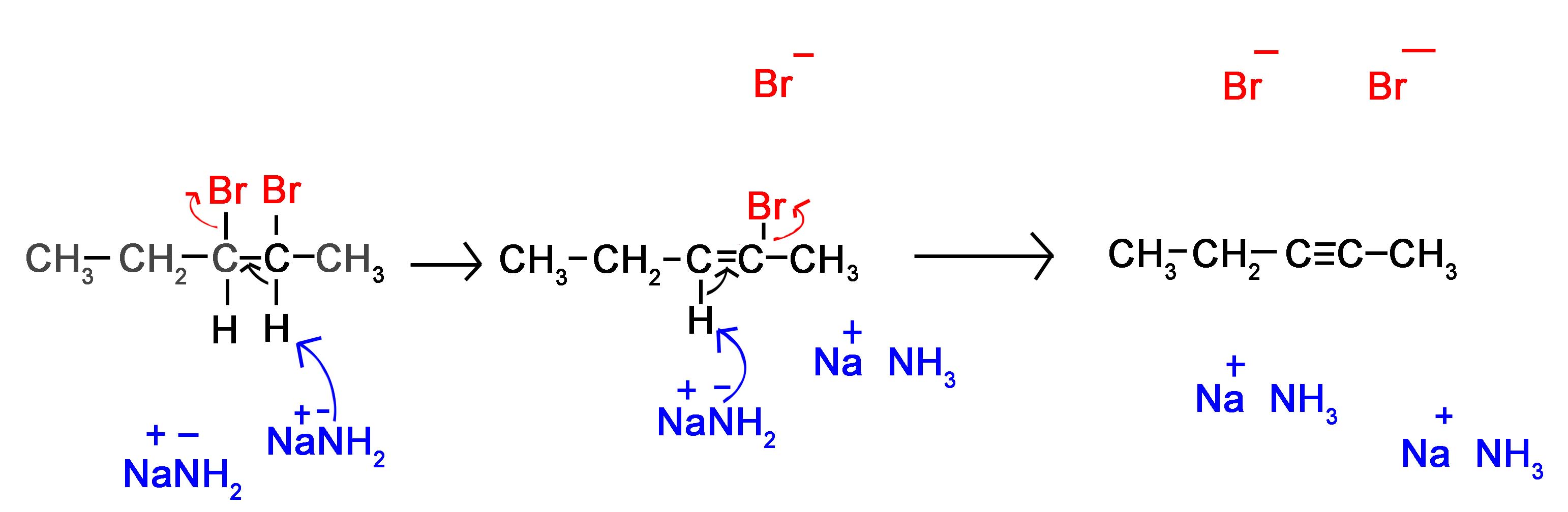 To synthesize alkynes from dihaloalkanes we use dehydrohalogenation. The majority of these reactions take place using alkoxide bases, such as sodamide in liquid ammonia, above, (other strong bases can also be used, such as Zn with high temperatures). This combination results in the majority of the product being from the E2 mechanism. Recall that the E2 mechanism is a concerted reaction (occurs in 1 step). However, in this 1 step there are 3 different changes in the molecule. |
|
Return |