Organolithium reagents react with cuprous iodide to give a lithium dimethylcopper reagent, which is referred to as a Gilman reagent. Gilman reagents are a source of carbanion like nucleophiles similar to Grignard and Organo lithium reagents. However, the reactivity of organocuprate reagents is slightly different and this difference will be exploited in different situations. In the case of α, β unsaturated carbonyls organocuprate reagents allow for an 1,4 addition of an alkyl group.
The coupling of Giman reactions with organochlorides, organobromides, and organoiodides is useful in organic synthesis because it forms a carbon bond. During the reaction one of the alkyl groups from the Gilman reagent replaces the halogen for the organohalide.
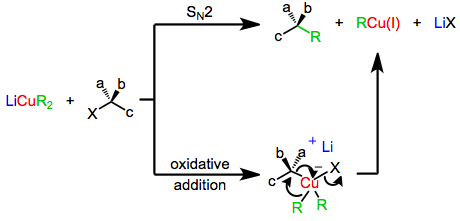
The mechanism of nucleophilic substitution by lower-order organocuprates depends in a profound way on the structure of the substrate, organocuprate, and reaction conditions. Early evidence suggested that a direct SN2 displacement was occurring; however more recent results suggest that invertive oxidative addition of copper(I) into the carbon-leaving group bond takes place, generating a copper(III) intermediate which then undergoes reductive elimination to generate the coupled product. Both of these mechanisms predict inversion at the electrophilic carbon, which is observed in a number of cases. On the other hand, experiments with radical traps and the observation of racemization during substitution suggest a radical mechanism.
|
