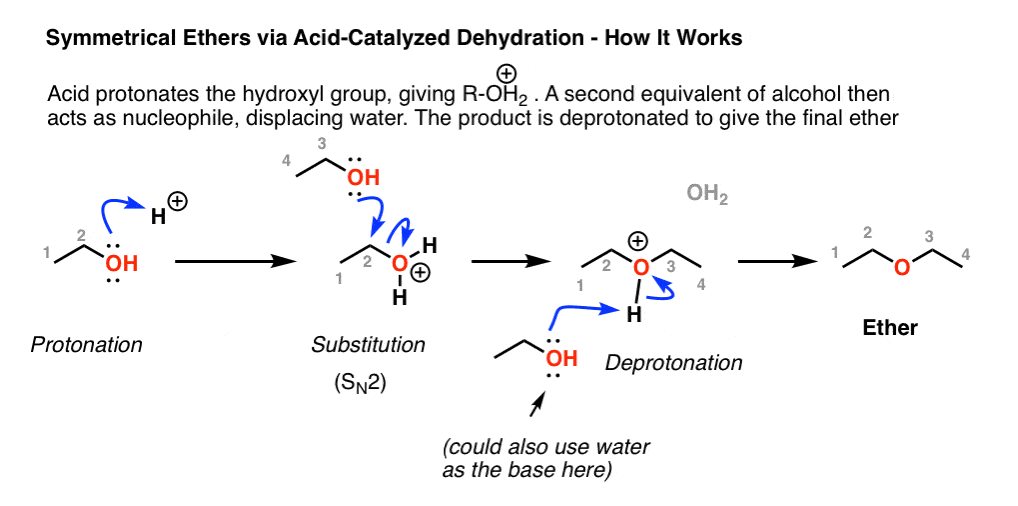
First of all, one equivalent of alcohol is protonated to its conjugate acid which has the good leaving group, OH2 (water, a weak base).
Next, another equivalent of the alcohol can now perform nucleophilic attack at carbon (SN2), leading to displacement of OH2 (water) and formation of a new C-O bond. This is an SN2 reaction.
The final step is deprotonation of the product by another equivalent of solvent (or other weak base), resulting in our ether product.
Conditions:
Care must be taken with the temperature. Too high and the primary product produced is an alkene.
|
