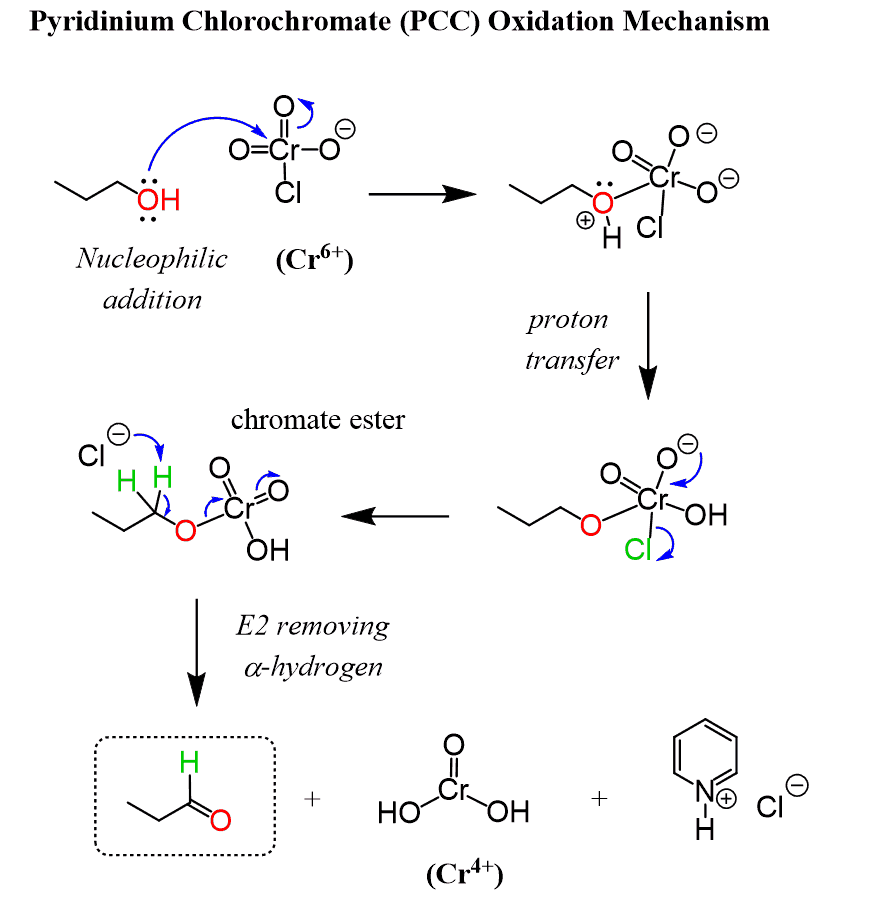
PCC is a Cr6+ salt formed between pyridine (C6H5N), HCl, and CrO3. It is soluble in halogenated organic solvents such as dichloromethane which allows carrying out the reaction in the absence of water. This also explains why PPC does not oxidize the aldehyde to a carboxylic acid as there is no water to turn the aldehyde into an aldehyde hydrate.
The reaction starts by converting the alcohol to its corresponding chromate ester, which then undergoes a deprotonation by a base to form a C=O double bond.
In the acid-base step, either the chloride ion or the alcohol can serve as a base to remove the hydrogen. Pyridine is certainly a better candidate for deprotonation, however, it is present in low concertation as a free-base in acidic conditions.
So far, all our attention was on the organic substrate, but, remember, this is an oxidation-reduction reaction and while the alcohol is being oxidized to a carbonyl, the Cr6+ is reduced to the corresponding Cr4+ species.
|
