Organic Synthesis
Formation
Up One Mechanism: HypoHalous |
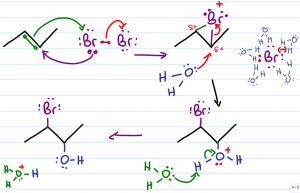 This shows the addition mechanism starting with a nucleophilic attack by pi bond on Bromine molecule, forming a cyclic bromonium ion bonded to both carbons. The more substituted carbon is then attacked by the nucleophile (H2O in the above picture), breaking one of the bonds to the bromine, then losing a hydrogen to a bromine ion. NBS can be used as a substitute for bromine. |