Organic Synthesis
Formation
Up One Mechanism: EsterHydrolysisBase |
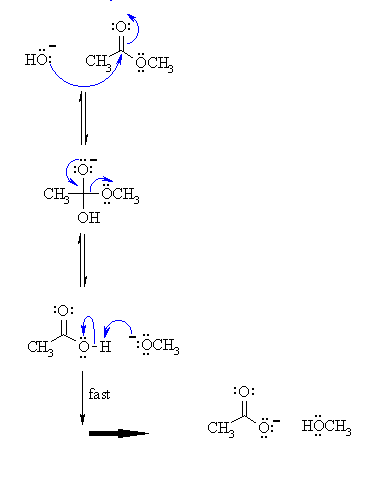 1) The hydroxide nucleophiles attacks at the electrophilic C of the ester C=O, breaking the pi bond and creating the tetrahedral intermediate. |
|
|
Organic SynthesisFormation
|