Organic Synthesis
Rxns
Up One Mechanism: OxidativeCleavage1 |
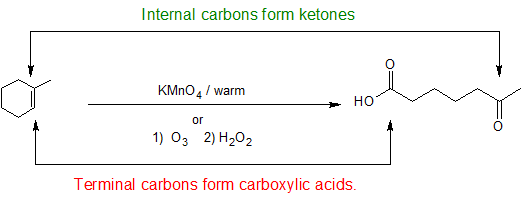 Nucleophilic attack by pi bond on an oxygen of the permanganate radical, triggering a cascade of pi bond electron shifts, resulting in an intermediate of another of the double bonded oxygens attacking the other carbon that had been part of the alkene's double bond. This intermediate splits the two carbons of the previous double bond, and if they have two other groups attached, are oxidized by the remaining permanganate to a ketone. If they have only one other bond, then they are oxidized to a carboxyl group. If the alkene being cleaved is a terminal alkene, then the longer non-terminal carbon is converted into a carboxylic acid, and the other carbon is oxidized to CO2. |