Organic Synthesis
NamedReactions
Up One Mechanism: SwernOxidation |
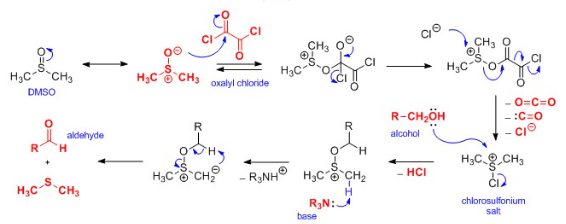 Dimethylchlorosulphonium ion is generated in situ from DMSO and oxalyl chloride. The reaction with an alcohol at -78°C leads to an alkoxysulphonium ion. |
|
|
Organic SynthesisNamedReactions
|