Mesityl Oxide and Phorone Synthesis
by Glenn Murray
|
Between my sophmore and junior years in chemistry, I was a lab assistant for the Columbus College Chemistry department. The supervisor for the lab assistants was a professor by the name of Al Osteen. Mr. Osteen would often run odd experiments. One such experiment was to add phosphorus pentaoxide to acetone - which resulted in some thick brown sludge along with the remaining acetone. He pointed out that it also had the smell of some flower. A smell none of us could pin down. Mr. Osteen left for greener pastures not long after that but the puzzle remained with me. I parlayed that mystery into a senior project several quarters later to find out what all was in the mixture. Adding phosphorus pentaoxide to acetone triggers a series of Aldol condensations, producing a large number of products. I separated perhaps 6 individual compounds, identifying two: Diacetone alcohol (4-hydroxy,4-methyl,pentan-2-one) and Mesityl oxide, but I never ID'ed the floral smell. It was one of those mysteries that stuck with me. Though I didn't go into chemistry as a profession, it always remained my first love, specifically organic chemistry with a strong emphasis on organic synthesis. Forty five years later, while perusing a book on organic synthesis I'd picked up from Amazon (Organic Syntheses - Vol I), the synthesis for Mesityl oxide caught my eye. As I read the procedure, I noticed a mention of a by-product called Phorone. In all my years in organic I'd never heard of phorone so I looked it up on wikipedia. I was dumbstruck by a single line, casually mentioned in the description: "is a yellow crystalline substance with a geranium odor". I discovered the identity of my mystery compound. I tried to track down Mr. Osteen, only to find out he'd died three years earlier. I wasn't able to let him know I'd solved the mystery. Not being able to inform him of my discovery, I decided to try a synthesis of phorone. A search of the web brought me to a paper, Novel Aspects in the Preparation of Phorone by Maria Koieczny and George Sosnovsky at the University of Wisconsin-Milwaukee, published January 4, 1978. It provided a good procedure for the synthesis of phorone and mesityl oxide. These are the syntheses for mesityl oxide and phorone (click pic for mechanism) 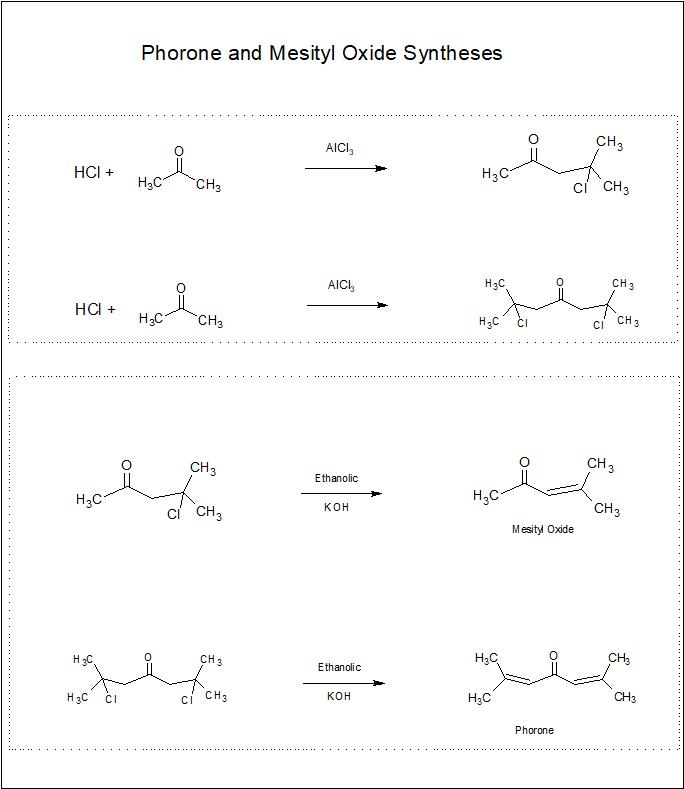
Procedure
|
Actual Process Used
|
|
|
"Meditation is not a means to an end. It is both the means and the end." - Krishnamurti |