Glaze CoTE Boric Oxide Project
Introduction and Purpose
This project was first suggested by Ron Roy. I had conversed with him about glazes over email. Ron Roy co-wrote one of the seminal books on glazes for cone six pottery, Mastering Cone 6 Glazes. I was to mix, construct, and fire the glaze rods to be tested, and Ron would do the invaluable step of grinding them down and measuring the thermal expansion in a dilatometer. What follows is that collaboration.
Explanation of glaze thermal expansion reality/computation can be simplified to the weighted sum of expansions of the various oxides the glaze is composed of when fired. A further simplification, assumed by ceramicists, is each oxide's contributed thermal expansion is linear throughout the proportions it can take in a glaze.
This project concerns where that assumption fails, specifically the case of higher percentages of lithium oxide and boric oxide. The thrust of this research is to investigate where thermal expansion changes from a negative to positive with changes in boric oxide content. There is some literature suggesting this occurs once Boron exceeds 14 to 16%.
The mechanism proposed in the literature, for this expansion reversal is a change from tetraborate to diborate ring formations in the boro-alumino-silicate glass matrix but confirmation of this is beyond the scope of this research.
Raw Materials
The glaze to be tested will be composed of raw materials, frits, and chemicals; the composition of which is listed below.
- Boric Acid(Optibor TG ) Accuracy Confidence: High
B2O3 56.5 LOI 43.5
- Feldspar G-200 HP (Imerys G-200 HP can be found here) Accuracy Confidence: Med-High
K2O 13.2 Na2O 1.52 CaO 0.750 Fe2O3 0.900 Al2O3 18.2 SiO2 65.9 LOI 0.160
- Frit Ferro 3134 (DigitalFire's assay of F3134) Accuracy Confidence: High
CaO 20.10 Na2O 10.30 B2O3 23.10 SiO2 46.50
- Whiting (Ground limestone/marble) Accuracy Confidence: Medium-high
Based on the Huber Q-325 composition; the computed Whiting composition used is
CaO 54.14 MgO 0.96 SiO2 1.0 LOI 43.9
- EPK Kaolin (Edgars Minerals, Inc.) Accuracy Confidence: Med High
SiO2 45.73 CaO 0.18 Al2O3 37.36 MgO 0.098 Fe2O3 0.79 Na2O 0.059 TiO2 0.37 K2O 0.33 P2O5 0.236 LOI 13.91
- Talc (Texas Talc)
Amtal C-98 is what is used in this project. Below, Amtal CB 27:73 is a mixture of 73% uncalcined Amtal C-98 and 27% calcined Amtal C-98 (not sold). The percents of all three are listed below
Amtal C-98 Calcined CB 27:73 SiO2 54.3 60.76 56.0 MgO 29.5 33.01 30.4 CaO 3.5 3.92 3.6 Al2O3 0.45 0.50 0.5 Fe2O3 0.45 0.50 0.5 TiO2 0.9 1.01 0.9 K2O 0.27 0.30 0.3 LOI 10.63 0.00 7.8
- Silica (US Silica - SIL-CO-SIL 52) Accuracy Confidence: High
SiO2 99.64 CaO <0.01 Al2O3 0.20 MgO <0.01 Fe2O3 0.026 Na2O <0.01 TiO2 0.02 K2O 0.01 LOI 0.01
Recipes
|
The base recipe is the Ron Roy/John Hesselberth "Mastering Cone 6 Glazes" Licorice glaze recipe without the colorants (Glossy Base Glaze 2, pg 96), with G200HP feldspar substituted for Custer, then the rest adjusted compensated for. Rather than starting with the percentage of the ingredients used in Glossy Base Glaze 2 (GBG2), I targetted developing a recipe coming close to getting the same oxide unity composition. This circumvents problem of differing of ingredient compositions, seen between locations and mine lots. Getting the sum of alkali oxides (K2O, Na2O) close to the same and the sum of the alkali earth (CaO, MgO) the same was more important that each separate flux oxide the same.
These are the resulting glazes:
The fired compositional breakdown for the Glossy Base Glaze 2:
Endpoint RecipesThe base glaze (GBG2) is used to prepare the 12% and 18% recipes.
MixturesThe mixes creating the target 12%, 14%, 15%, 16%, and 18% using the 12% and 18% recipes as the base, with the following proportions:
|
Procedure
|
The glaze picked as the base for this test is Mastering Cone 6 Glazes: Glossy Base Glaze 2, then modified to use G-200HP instead of Custer Feldspar. To the glaze, boric acid was added to increase the boric oxide content to 12 and 18%, respectively. The glaze is expected to be suspended in a non-aqueous, non-polar solution to prevent the boric acid from dissolving in the glaze. The glaze base is to be mixed and sieved through an 80 mesh sieve, then amounts measured to compound the 12% and 18% batches. Once the two batches are made, three more batches can be prepared, 14, 15, and 16%, as 2:1, 1:1, 1:2, mixtures of the 12 and 18% batches. This allows for 12, 14, 15, 16, and 18% batches. The 15% batch is being run because of its closeness to the turning point in CoTE. The five batches will be mixed with a non-aqueous, non-polar solvent and allowed to build up enough glaze to fill the glaze boats such that a 3/8 inch by 3/8 inch by 3 1/2 inch bar of glaze can be produced for each compositional mix. The boats will be fired to cone 6, soaked at that temp for 20 minutes, then soaked at 50 degrees below cone 6 for 30 minutes, cooled to 300 degrees below cone 6, soaked for one hour, then cooled normal. If the bars are not clear of bubbles or inclusions, they may need to be fired once again. The labeled bars will then be sent to Ron Roy for measuring actual expansion for the bars using a dilatometer. |
Steps:
|
Results
|
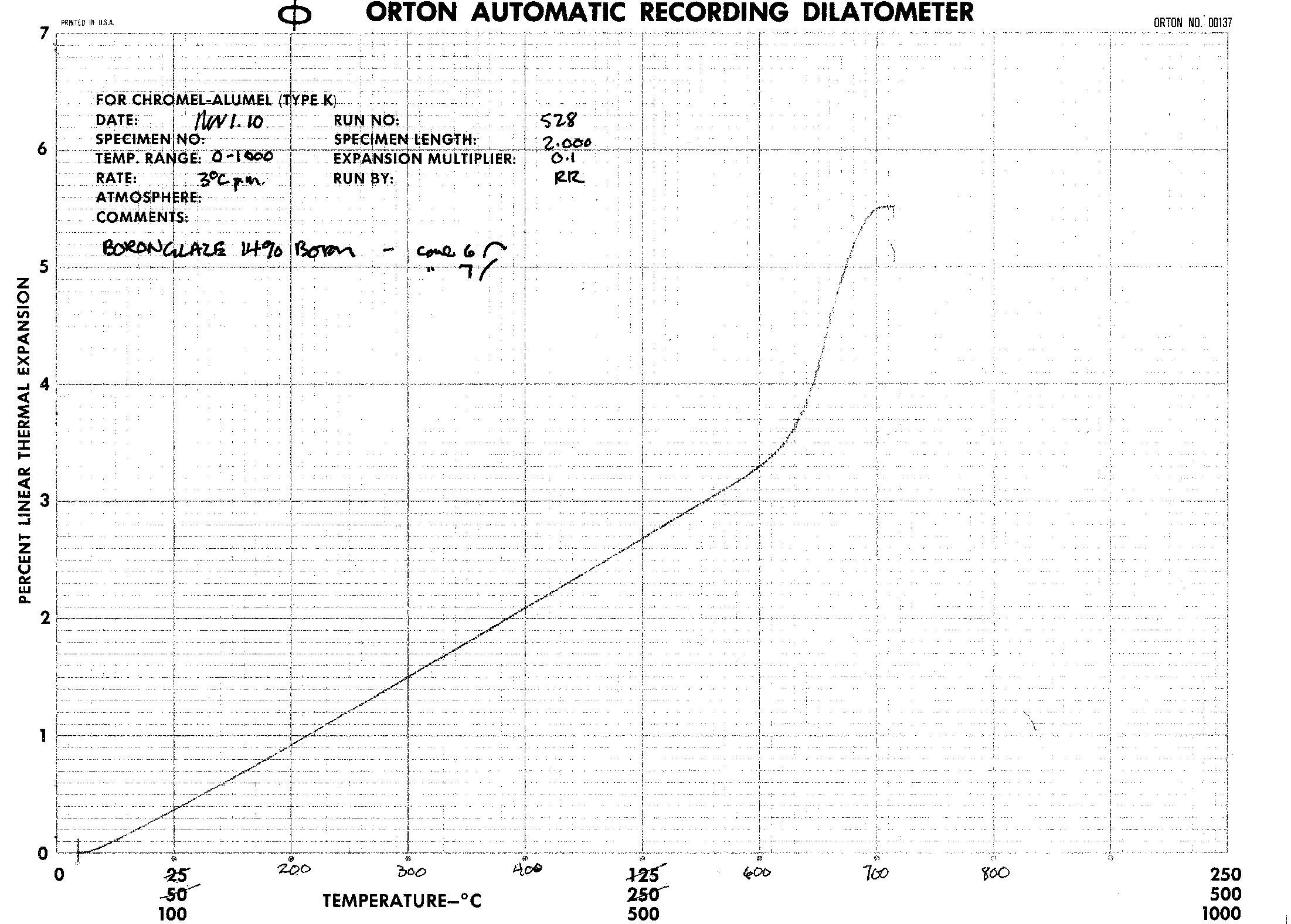
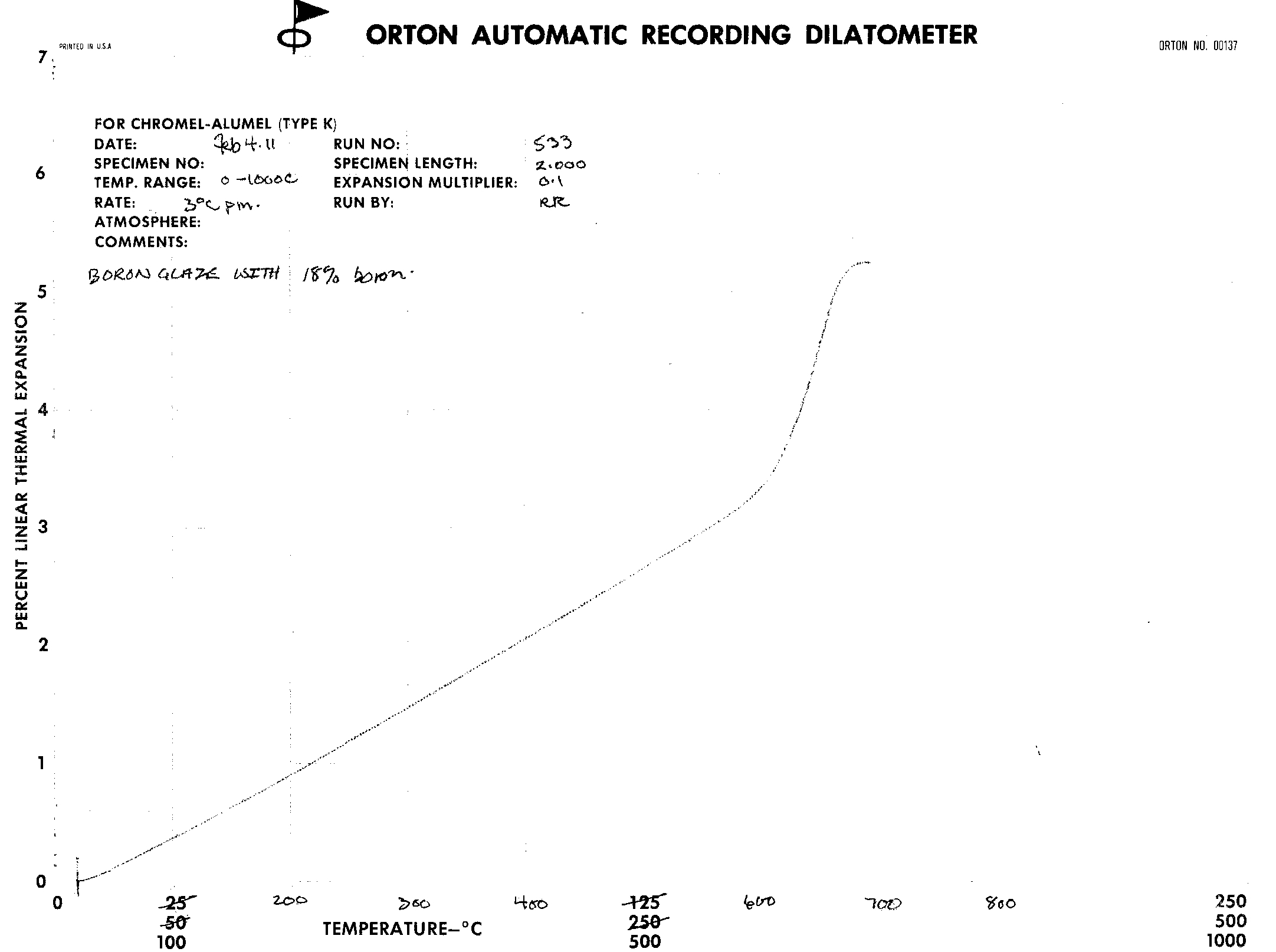
The data and results are in the form of dilatometer graphs. These graphs seem to show little difference in the Coefficient of Linear Thermal Expansion as the concentration of boric oxide proceeds from 12% to 18%. |
|
|
|
|
|
|
|
|
Analysis and Conclusions
|
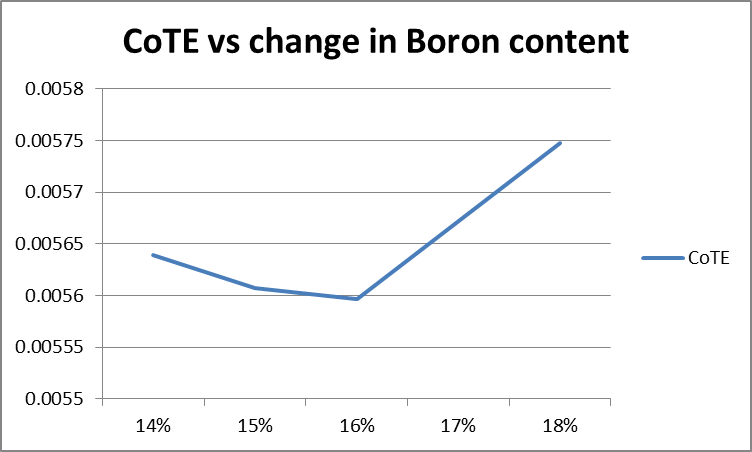
The thermal expansions were gotten for the following Boron formulations: 14%, 15%, 16%, and 18%. They are derived from the fairly linear portion of the graphs in the range of 20 to 540 degrees Celcius.
From the above graph, the thermal expansion decreases as the Boron content increases, as expected, until 16% Boron content. Between 16 and 18% Boron, the change in thermal expansion reverses direction. Determination of the CoTE was from the grainy images of the dilatometer graph output, therefore was not as precise as desirable. The initial results indicate a reversal of CoTE occurred, with increasing Boron. That said, a single set of tests are a poor basis for deriving conclusions. Another set of tests would increase the confidence of the conclusions. A good suggestion would be to use a different procedure for the incorporation of high levels of Boron. Use of a differing approach would help rule out any undiscovered errors inherent to this method. |